Probing the Equatorial Electronic Structure of Uranyl Ions
Comprehensive understanding of uranyl ions provides insight into chemistry of nuclear waste and uranium separation technologies
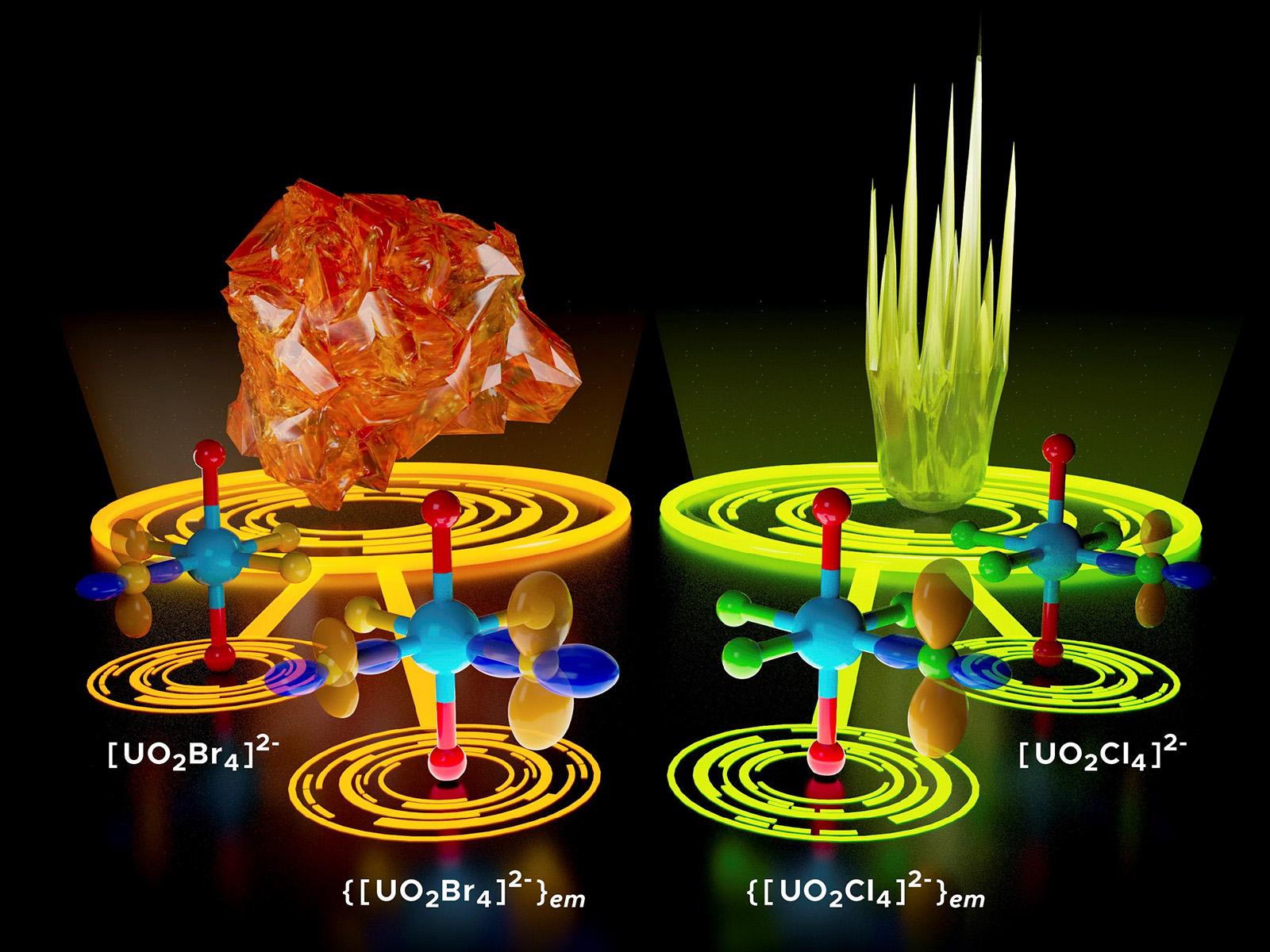
The metal-ligand interactions of uranyl complexes are an important determinant of the chemistry of the complex.
(Image by Cortland Johnson | Pacific Northwest National Laboratory)
The Science
Actinide elements are immensely important due to their roles in nuclear energy and national security technologies. Their chemistry displays a complexity that defies explanations developed for lighter elements. Actinyl complexes have been a valuable model for studying the chemical bonding of the actinide elements. Previous studies mainly focused on the axial metal-oxygen bond of actinyl complexes. In this work, the equatorial environment of two different uranyl complexes was directly probed by measuring the electric field gradient (EFG) tensors of ligands in the perpendicular plane. This research expands the maps of the complex’s electronic environment beyond the covalent U-O bonds and thus provides a more comprehensive picture of the electronic structure.
The Impact
Uranyl is a prevalent form of uranium in nature and in nuclear waste. It can also form compounds that are important for catalysis, ion-exchange, and absorption applications. This research provides a comprehensive picture of the electronic structure of uranyl compounds by investigating the equatorial plane. These results advance our understanding of the chemistry of this complex by revealing insights into how equatorial ligands can activate actinyl oxygens and promote the reactivity of this normally inert moiety. These results can help us understand the physics and chemistry of actinide-containing systems, such as next-generation nuclear fuels, novel superconductors, organometallic clusters, and radioactive waste.
Summary
The electronic structures of uranyl complexes were probed by measuring EFG tensors in the equatorial plane of the linear UO2 2+ ion. EFG tensors were measured using nuclear magnetic resonance and nuclear quadrupole resonance experiments and computed by relativistic Kohn−Sham methods with and without environment embedding for Cs2UO2Cl4 and Cs2UO2Br4. This approach expands the possibilities for probing the electronic structure in uranyl complexes beyond the strongly covalent U−O bonds.
This research lays the groundwork for future studies of the distribution of electrons between ligands and actinide metals. The analysis of EFG and shielding tensors shows that the magnetic parameters of ligand nuclei are sensitive and exacting guides toward accurate descriptions of metal-ligand bonding. Further work is under way to extend the approach of measuring local magnetic fields to additional sites in actinyl systems and to other actinide complexes. Numerous isotopes can serve as probes of local magnetic and electric fields by this general technique, including actinide nuclides, which would allow the construction of more comprehensive and accurate models of the electronic structure in 5f element systems.
Contact
Herman Cho
Physical and Computational Sciences Directorate, Pacific Northwest National Laboratory
hm.cho@pnnl.gov
Funding
This work was supported by the Department of Energy Office of Science, Basic Energy Sciences program, Division of Chemical Sciences, Geosciences and Biosciences, Heavy Element Chemistry program.
Published: February 3, 2022
Sergentu D-C, Gendron F, Walter ED, Park S, Capan C, Surbella RG, Soderquist CZ, Hall GB, Sinkov SI, Autschbach J, Cho H. “Equatorial Electronic Structure in the Uranyl Ion: Cs2UO2Cl4 and Cs2UO2Br4.” Inorganic Chemistry, in press (DOI: 10.1021/acs.inorgchem.1c02832)